CLIA-WAIVED DRUG TESTS
Which CLIA Waived Drug Test Do You Need?
What Does CLIA Waived Drug Test Mean?
Have you ever heard someone say: “This test is CLIA-Waived,” but do not really know what that means?CLIA waived drug tests are a type of diagnostic test that are approved by the Centers for Medicare and Medicaid Services (CMS) for use in waived testing sites. These tests are designed to be simple and easy to use, with the goal of providing rapid results for a variety of diagnostic purposes. Clia drug test can still be used at a non clia waived testing site but then they are not able receive the reimburment potential but using the correct CPT codes. An example of a facility that can achieve clia waived status would be a pain managment clinic or a substance abuse treatment facility. As these clinics are treating pateints with licensed medical practitioners and providing medical diagnosis.
CLIA stands for Clinical Laboratory Improvement Amendments, which are a set of regulations that apply to all clinical laboratory testing performed on humans in the United States. These regulations establish standards for the quality and accuracy of laboratory testing, as well as the qualifications and training of personnel who perform the tests.In order to be classified as a CLIA waived test, a diagnostic test must meet certain criteria set forth by the CMS. These criteria include the level of complexity of the test, the potential risks associated with the test, and the level of training required to perform the test.CLIA waived drug tests are often used in settings such as hospitals, clinics, physician offices, and other healthcare facilities. These tests may be used to detect the presence of drugs or other substances in a patient's body, as well as to monitor the effectiveness of certain medications. Overall, CLIA waived drug tests play an important role in the diagnosis and treatment of various medical conditions. They provide fast, accurate results and can be performed by a wide range of healthcare professionals, making them a valuable tool in the healthcare industry.
What are the Requirements For a CLIA-Waived Drug Screen
Waived tests include test systems cleared by the FDA (Federal Food and Drug Administration) for home use and those tests approved for waiver under the CLIA (Clinical Laboratory Improvement Amendment Program) criteria. Although CLIA requires that waived tests must be simple and have a low risk for erroneous results, this does not mean that waived tests are completely error-proof.When using a CLIA-Waived drug test, it’s important to follow exact instructions, avoiding anything that can possibly alter the results. In just a few minutes you will be able to get quick results. The accuracy of your results will depend on the correct administration of the test and the quality of the test. At Halux Diagnostic, we offer a wide variety of high quality clia waived drug testing products that can be used for your business. All of our drug tests have been through extensive trials, making them have a 99% level of accuracy.
CLIA requirements apply to the clinical use of drug testing. This includes any entity that performs tests on materials derived from the human body in order to provide an assessment of the person’s health, information for diagnosis, and treatment or prevention of any impairment or disease. Any company performing a test for these set of purposes are considered by CLIA to be a laboratory, and must abide by CLIA regulations by registering with the CLIA Program if they want to receive the benefits.
What are CPT Codes for CLIA-Waived Tests?
Click here to see a complete list- Note: this is only for informational purposes and may not accurately represent current CMS CPT Codes. Make sure to verify the status of your waived tests and CPT Code prior to implementing any testing. Drugs of Abuse (Use G0477QW for visually read devices or G0478QW for instrument read devices).Why Customers Use Our CLIA Waived Drug Test
Buy CLIA-Waived Drug Test Cups!
Are you looking for a reliable and accurate drug testing solution? Look no further than clia waived drug tests! Our FDA-approved, CLIA-waived drug tests provide employers or medical facilities with the peace of mind that comes with knowing their screening results are true and accurate. With our state-of-the-art technology, these tests offer quick turnarounds and sample collection procedures that take only minutes to complete. Plus, they’re easy to use and require no specialized training or additional machinery or devices. Perfect for employers and Clinics who need to conduct onsite screenings quickly and easily, our clia waived drug tests let you get through the process faster while providing you with trusted results every time. Get started today - choose clia waived drug tests for your next round of screenings!
When it comes to critical decisions regarding drug testing, employers need to be sure they are using a reliable product that is approved by the highest authorities. CLIA Waived Drug Tests provide peace of mind with FDA and CLIA approved drug testing options. Thoroughly vetted for accuracy and dependability, these tests have gone through rigorous quality assurance processes to ensure accurate results time after time. With customizable test panels, employers can get the information they need quickly and easily. Quality control is at the top of the list for many employers and medical facilities, so turn to CLIA Waived Drug Tests for accurate results you can count on from Halux Diagnsotic!
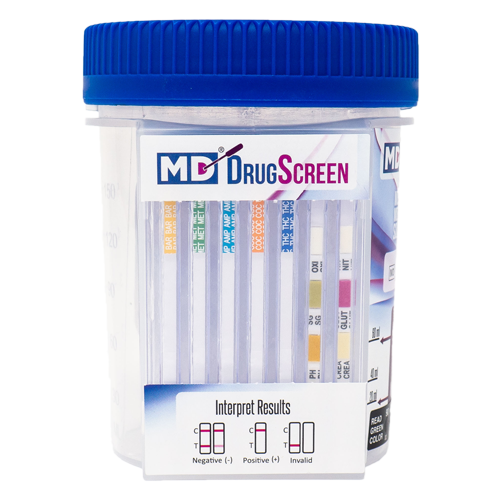
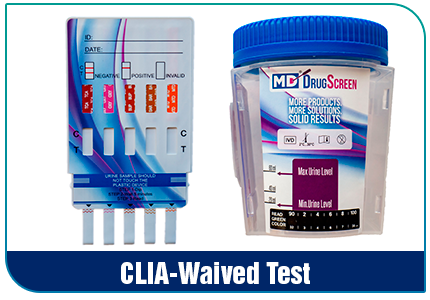
How to Read Clia Waived Drug Test Cup
Clia waived drug test cup is a simple and convenient way to perform a drug test. These cups typically include one or more panels of tests, each designed to detect a specific drug or group of drugs. The results are easy to read and interpret, making them a popular choice for many workplaces, medical facilities, and other settings.
Here's a step-by-step guide on how to read a CLIA waived drug test cup:
Read the instructions: First familiarize yourself with how to use the test. These will give you important information about how to perform the test correctly and how to interpret the results. Once knowing how to perform the test and doing a few it will become very easy.
Collect the specimen: This will involve having the person being tested provide a urine sample. The results will appear directly in the side of the test cup.
Wait: This will vary depending on the test cup, but is typically a few minutes.
Review the Results: to the control line(s) and the test line(s). The control line(s) will indicate whether the test is working correctly, while the test line(s) will show the results of the test.
Interpreting results: If the control line appears but the test line(s) does not, this means that the test is negative. If the control line appears and the test line(s) appears, this means that the test is positive for that specific drug.
Record the results: This is important for legal, medical or work-related purposes. It's important to note that CLIA waived test cups are still not as sensitive or specific as laboratory-based tests. Positive results should always be confirmed with a more sensitive test, such as a lab-based test.


Why Would a Business Use a CLIA-waived Test For Drug Screening?
CLIA-waived drug tests (Clinical Laboratory Improvement Amendments – CLIA) utilize very simple and concise procedures that are substantially accurate as to determine the percentages of false or negative results and pose no reasonable risk of harm to the tested person in the event the drug test were to be erroneously performed. These types of drug tests are approved and cleared by FDA as suitable for home and business use. Additional details about CLIA-waived drug test by their type of application are published by the FDA and include information and summary of the data submitted by different manufacturers who have applied to validate the determination that a CLIA-waived drug test system meets CLIA statutory criteria for waiver, and FDA’s justification for approving their CLIA-waived application.These conclusions and decisions by the FDA provide information that is useful for manufacturers preparing future CW submissions and allow the public to be informed.
Reference and resources:
Information about CLIA Waivers
Clinical Laboratory Improvement Amendments