12 Panel Round Drug Test Cup-Urine Drug Test Cup FDA-Cleared & CLIA-Waived
May 9, 2019Covid Home Test Kits
January 23, 2023Covid-19 Rapid Test Kit
$450 per box of 25 Tests *Now In Stock*
Call 407-680-2209 to place order
*Now accepting Orders For Qualified Medical Professionals*
⏺ 25 tests per box
⏺ For Professional use only
⏺ Rapid Results in as little as 7-10 min
⏺ Detection Window IgM: Symptomatic 3-5 days, Asymptomatic 7 days
What is a Covid-19 Rapid Test Kit
A Covid-19 rapid test kit is a medical device used to detect the presence of antibodies in an individual’s blood. The 2019-nCoV(COVID-19) IgG/IgM Rapid Test is the qualitative detection of coronavirus N-Protein IgM / IgG antibodies in human whole blood, serum or plasma. It is intended to be used by the professionals as a screening test and as an aid in the diagnosis of infection with coronavirus(COVID-19). This test provides only a preliminary test result. Therefore, any reactive specimen with the COVID-19 IgG/IgM Rapid Test Cassette (Whole Blood/Serum/Plasma) must be confirmed with alternative testing method(s) and clinical findings.
This type of testing can provide results within minutes, making it a convenient and efficient way to determine if someone has been exposed to the virus. The kits are designed for use by healthcare professionals or at home with minimal training required. The most common type of Covid-19 rapid test kit uses immunochromatography technology which detects IgG and IgM antibodies that are produced by the body when it is exposed to SARS-CoV-2, the virus responsible for causing COVID-19. These tests work by taking a small sample of blood from an individual’s fingertip and then applying it onto a special strip that contains antigens specific to SARS-CoV-2. If antibodies are present in the sample, they will bind with these antigens and produce visible lines on the strip indicating positive results for exposure to COVID 19.
Covid 19 rapid test kits offer several advantages over traditional laboratory testing methods such as PCR (polymerase chain reaction) tests which require specialized equipment and take days or weeks before results can be obtained. Unlike PCR tests, these kits do not need any additional lab processing so they can be administered quickly without having to wait for lab technicians or expensive machines. Additionally, since no needles are involved in this process there is less risk of infection transmission compared with other forms of testing such as nasopharyngeal swabs which require inserting long tubes into individuals’ noses or throats.
Covid 19 rapid test kits have become increasingly popular due their convenience and accuracy but should only be used under certain circumstances where time is critical factor such as during outbreaks or epidemics where quick diagnosis could save lives by allowing people who have been infected with coronavirus access treatment sooner rather than later thus reducing its spread throughout communities worldwide. It’s important however that anyone using one should always follow instructions carefully including proper storage procedures since incorrect usage may lead inaccurate readings leading false positivesnegatives which could put both patients’ health at risk as well those around them if appropriate precautions aren’t taken afterwards .
How to Protect Yourself and Others as Recommended by the CDC
- The best way to prevent illness is to avoid being exposed to this virus.
- The virus is thought to spread mainly from person to person
- Between people who are in close contact with one another (within about 6 feet).
- Through respiratory droplets produced when an infected person coughs, sneezes or talks.
- These droplets can land in the mouths or noses of people who are nearby or possibly be inhaled into the lungs.
- Some recent studies have suggested that COVID-19 may be spread by people who are not showing symptoms.
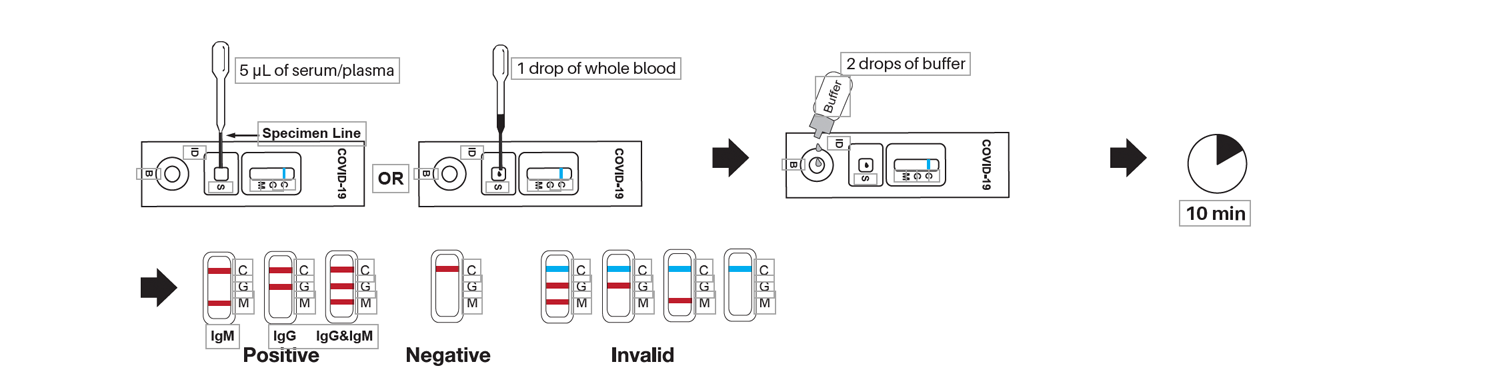
Directions
Disclosure:
The test has not been reviewed by the FDA and results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status.
CLICK Here For COVID-19 IgG IgM Performance characteristic
Covid -19 Rapid Test Kits for Healthcare
Covid-19 rapid test kits are a critical tool for healthcare providers in the fight against the coronavirus pandemic.
These tests allow medical professionals to quickly and accurately detect the presence of antibodies associated with Covid-19, helping them diagnose patients more quickly and effectively. The most common type of Covid-19 rapid test kit is an IgGIgM antibody test, which looks for both immunoglobulin G (IgG) and immunoglobulin M (IgM) antibodies in a patient’s blood sample. If either or both types of antibodies are present, it indicates that the person has been exposed to the virus at some point in their life. This information can be used by healthcare providers to determine if further testing or treatment is necessary. These tests are typically conducted using finger prick samples taken from patients who have recently experienced symptoms consistent with Covid-19 infection such as fever, coughing, difficulty breathing or fatigue. The results of these tests can be obtained within minutes and provide valuable insight into whether a patient should receive additional care or not. In addition to providing quick results, these Covid-19 rapid test kits also offer several advantages over traditional laboratory testing methods such as PCR testing. For instance, they require no special equipment or expertise on behalf of healthcare workers administering them; they can be performed anywhere without access to a lab; and they cost significantly less than other forms of diagnostic testing available today.
SInce these tests do not rely on detecting active viral particles like PCR tests do – instead relying solely on antibody detection – they may prove useful even after someone has recovered from Covid-19 infection since residual levels of antibodies may remain detectable long after recovery has occurred. This could help identify people who have already had exposure to the virus but did not experience any symptoms during their illness – something that would otherwise go undetected by traditional laboratory methods alone . Overall ,Covid – 19 rapid Test Kits for Healthcare offer many benefits including fast turnaround times , ease of use , low cost ,and ability to detect past exposures .
As we continue our efforts towards containing this pandemic ,these tools will play an important role in helping us achieve this goal .
How Does a Rapid Covid- 19 Test Work
A rapid Covid-19 test is a type of diagnostic test that can detect the presence of antibodies to the virus in a person’s blood.
This type of testing is used to determine if someone has been infected with the virus, and whether they have developed immunity against it.
The most common form of this test is an immunoassay, which uses specific antigens (proteins) from the virus to identify antibodies in a sample of blood.
What Does It Test For?
The rapid Covid-19 test detects two types of antibodies: IgG and IgM.
IgG antibodies are produced by the body after infection with SARS-CoV-2, and indicate that an individual has had previous exposure to the virus.
On the other hand, IgM antibodies are produced early on during infection and may remain present for several weeks or months afterwards.
How Accurate Is It?
Rapid Covid-19 tests are generally considered very accurate when compared to other forms of testing such as PCR tests or antigen tests.
Studies have shown that these tests can detect up to 97% accuracy for both IgG and IgM antibody detection when performed correctly according to manufacturer instructions.
Additionally, false positives are rare with this type of testing due its specificity for detecting only SARS-CoV-2 related proteins rather than general viral proteins found in other viruses such as influenza or rhinovirus infections.
How Quickly Can Results Be Obtained?
Rapid Covid-19 tests typically provide results within 15 minutes after collection making them ideal for quick diagnosis in situations where time is critical such as at airports or healthcare facilities where people need immediate answers about their health status before entering public spaces or receiving medical care respectively .
Furthermore , these types of tests do not require any special equipment meaning they can be administered almost anywhere without having access specialized laboratory settings .
Are There Any Limitations To These Tests ?
Yes , there are some limitations associated with rapid covid – 19 testing .
First , because these types of tests rely on identifying specific antigens from SARS – CoV – 2 , they may not be able to accurately detect infections caused by variants which could lead to false negatives .
Secondly , since these types of diagnostics measure levels over time instead one single point measurement like PCR does it cannot confirm recent exposure nor rule out active infection .
Lastly , although uncommon false positives may occur due incorrect handling procedures during administration so it’s important follow all manufacturer guidelines closely when performing this type diagnostic testing
What is the Difference Between an Igm and Igg antibody?
The COVID-19 IgGIgM Rapid Test is a blood test that detects the presence of antibodies against the virus. It can help to determine if someone has been infected with SARS-CoV-2, the virus that causes COVID-19. The test looks for two types of antibodies:
Immunoglobulin M (IgM) and immunoglobulin G (IgG).
What is Immunoglobulin M (IgM)?
Immunoglobulin M (IgM) is an antibody produced by the body in response to a new infection or antigen. It is usually found in large amounts shortly after infection and then decreases over time as other immune responses take over.
IgM helps fight off infections by binding to antigens on bacteria, viruses, and other foreign substances so they can be destroyed by white blood cells.
This type of antibody typically appears within 5–10 days after initial exposure to an antigen or pathogen and remains present for up to 3 months afterwards.
What is Immunoglobulin G (IgG)?
Immunoglobulin G (IgG) is another type of antibody produced by the body in response to an infection or antigen but it takes longer than IgM for it to appear in detectable levels following exposure – usually about 10–14 days later.
Unlike IgM which declines quickly once it has done its job, IgG remains at high levels even after symptoms have gone away because it provides long term protection from reinfection with that particular pathogen or antigen.
In addition, when someone has had a previous encounter with a specific pathogen their body will produce more IgG faster upon subsequent exposures due its “memory” effect which helps provide better immunity against future infections from similar pathogens or antigens..
How do these Antibodies Differ?
The main difference between these two types of antibodies lies in how quickly they are produced following initial exposure; while both are important components of our immune system’s defense mechanism against pathogens, each plays different roles depending on when they are present during an infection cycle.
For example, since IgM appears earlier than IgG it plays a key role in fighting off early stages of disease whereas later on during recovery phase when most symptoms have subsided but there may still be lingering viral particles around – this is where having higher concentrations of protective anti-bodies like those provided by increased production of IgGs come into play as well as providing immunity against future reoccurrences from similar agents.
Product Insert and Performance Data
Lateral Flow COVID-19 IgG-IgM PI Product Insert
COVID-19 IgG IgM Performance characteristic
You must be logged in to post a review.



Reviews
There are no reviews yet